Conference Series LLC Ltdinvites all the participants from all over the world to attend "21st International Conference on Radiooncology & Combinatorial Cancer Therapies" during May 30- 31, 2019 Florida,USA which includes prompt keynote presentations, Oral talks, Poster presentations, Young research forums and Exhibitions.
Details of Radiooncology Conferences 2019 in Florida USA:
The Theme of the Radiooncology 2019 is "Contemporary paradigms of Radiooncology and combination therapies" which covers the wide range of critically important sessions in the field of Oncology, Radiology, Cancer, and Imaging. This multidisciplinary multi-specialty oncology course will cover all aspects of cancer and radiology translational research. The goal of this meeting is to share the outstanding knowledge on the radiology and the novel technologies in Cancer treatment.
Each session of the meeting will be included with expert lectures, poster and discussions, join us to design sustainable development processes, innovations by which and how these strategies drive new policies, advances the business and sustainability in drug production for further health care protection of lives. We are glad to invite you on behalf of organizing committee to join us, where you are the decision maker for future.
Target audience:
Physicians & Physicists
Nurses
Dosimetrists
Radiation Therapists
Biologists
Dietitians
Radiographers
Naturopathic clinicians
Pain management practitioners
Mind-body therapists
Rehabilitation therapists
Scientists, Researchers for research groups and institutions
Professors, Deans, Directors, HOD's from universities
Cancer is a disease characterized by uncontrolled cell proliferation. Of the more than 100 types of cancer, lung, breast, and colorectal cancer account for nearly half of new cases per year in the United States. Lung cancer, which is highly correlated with cigarette smoking, is responsible for more deaths than any other form of cancer. Cigarette smoking and other environmental factors are associated with the majority of cancers. Increasing Patient Assistance Programs (PAP’s), growing government initiatives for cancer awareness, rising prevalence of cancer worldwide, and strong R&D initiatives from key players are the major factors fueling the growth of the cancer therapy market. The cancer therapy market was valued at USD 125730.7 million in 2017, is expected to register a CAGR of about 8.37% during the forecast period, 2018-2023. Cancer is one of the leading causes of morbidity and mortality, globally. According to World Health Organization (WHO), in 2015, cancer was the second leading cause of death accounting for 8.8 million deaths, worldwide, and the number of new cancer cases is expected to rise by about 70% over the next two decades. It has been identified that approximately 70% of deaths from cancer occur in low- and middle-income countries. Cancer rates could further increase by 50%, to 15 million new cases by 2020, as per the World Cancer Report. The growing incidence and prevalence of cancer and launch of novel cancer therapy, coupled with increasing awareness about cancer cure and treatment, are likely to drive the growth of the global cancer therapy market during the forecast period.
Additionally, increasing Patient Assistance Programs (PAP’s), growing government initiatives for cancer awareness, and strong R&D Initiatives from key players are anticipated to drive the market for global cancer therapies.
What makes the growth of Cancer Therapy market?
The annual growth rate in cancer drug costs skyrocketed from 3.8% in 2011 to 11.5% in 2015, at a constant exchange rate. Growth in the American market rose from 2.0% to 13.9% during the same time period. The United States now accounts for almost 45% of the global market for oncology therapeutics, up from 39% in 2011, owing to the strengthening of the US dollar and the rapid adoption of innovative, novel and newer therapies. In the United States, oncology drugs account for more than 11.5% of the total drug costs, up from 10.5% in 2011. Net price growth in the United States, on established branded oncology drugs, had averaged an estimated 4.8% in 2015, as compared to 6.4% invoice price growth. Factors, such as longer duration of cancer therapies, availability of novel agents, and use of combination therapy are contributing to the rising cost of cancer therapy. Thus, the rising cost of cancer therapies, coupled with the fluctuation of reimbursement policies for cancer treatment across the globe, is likely to impede the growth of the global cancer therapy market.
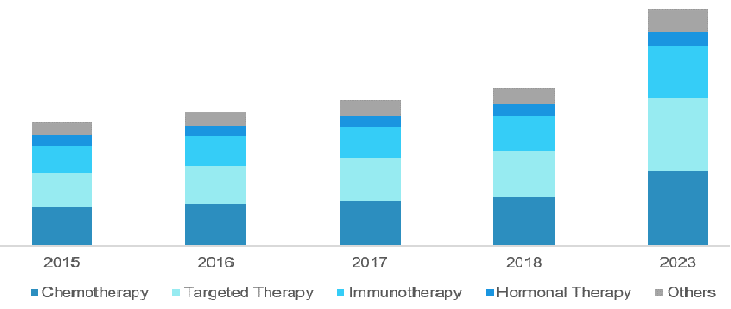
North America’s role in the Cancer Therapy Market
North America dominates the global cancer therapy market, owing to a high prevalence of cancer in the region because of a sedentary lifestyle, along with launch and growing adoption of novel cancer therapy in the region. USFDA is taking steps to enhance the growth of the cancer therapy market by approving drugs that are in clinical phase, thus, accelerating the clinical developments. Companies and research organizations are investing in R&D activities. Hence, the market is expanding at a faster pace.
Key Developments in the Cancer Therapy Market:
February 2018: Bristol-Myers Squibb Company announced cancer deal with Nektar Therapeutics. As per the deal, Bristol-Myers Squibb Company will pay USD 1.85 billion to Nektar Therapeutics. The partnership is built around the Nektar drug, NKTR-214, which will be tested in combination with Bristol’s immuno-oncology (IO) drugs Opdivo and Opdivo and Yervoy in 20 cancer indications across nine different tumor types, including melanoma, kidney, and lung cancers
January 2018: AstraZeneca and Merck & Co., Inc., received approval from US Food and Drug Administration, for Lynparza (olaparib), for use in patients with deleterious or suspected deleterious germline BRCA-mutated (gBRCAm), human epidermal growth factor receptor 2 (HER2)-negative metastatic breast cancer.
The major players include Amgen, AstraZeneca, Bristol-Myers Squibb, Bayer AG, F. Hoffmann-La Roche Ltd., GlaxoSmithKline PLC., Johnson & Johnson., Merck & Co. Inc., Novartis AG, Pfizer Inc., among others.






